Seung Hyun Ryu
Researcher in Seoul National University College of Medicine
Seoul National University
Biography
Welcome! I’m Seung Hyun Ryu, a researcher at Seoul National University College of Medicine. I work in the Laboratory of Neural Functions and Bio-imaging, focusing on neural signaling mechanisms and trafficking pathways—specifically synaptic biogenesis and presynaptic homeostatic plasticity (PHP). I authored a research paper in Autophagy (2025) and co-authored publications in PNAS (2021, 2023), Nature Methods (2023), and many others. My work has been recognized with the Fellowship for Fundamental Academic Fields and an IBRO World Congress 2023 Travel Grant Award. I’m always eager to connect and discuss with neuroscientists, biologists, and also neuro-folks. Feel free to reach out!
Download my CV.
- Neuroscience
- Molecular and cellular biology
- Physiology
-
M.S. in Interdisciplinary Program in Neuroscience, 2023
Seoul National University
-
B.E. in Control and Instrumentation Engineering and Biomedical Engineering, 2020
Korea University
Skills
DNA Cloning, DNA Prep, AAV Production, CRISPR/Cas9-Based Gene Editing
Primary Neuron Culture, Cell Transfection, Immunocytochemistry
SDS-PAGE(with Staining), Western Blot, Immunoprecipitation
In-utero Electroporation, Immunohistochemistry
Morphological Imaging, Live Cell Imaging, Physiological Imaging(iGluSnFR, GCaMP, pHluorin…), Super Resolution Imaging(STORM, ExM…)
Professional Experience
Honors & Awards
(Poster - Image Based Doorlock System)
(Poster - Self Healthcare Device Using EOG Measurement)
(Poster - Sound Activated Multi Color LED Cube)
Recent & Upcoming Talks
Talks & Presentations
Featured Publications

Neuronal autophagosome formation at distant presynaptic sites relies on ATG9A trafficking, a process mediated by AP-4 at the trans-Golgi network (TGN), but the molecular mechanisms governing its sorting for presynaptic delivery have remained elusive. Here, we uncover an unexpected role for SCAMP5, a key regulator of synaptic vesicle dynamics, in orchestrating presynaptic macroautophagy/autophagy through its actions at the TGN. SCAMP5 depletion severely impairs autophagosome formation at presynaptic boutons. Mechanistically, we identify SCAMP5 as a novel binding partner of PI4KB/PI4KIIIβ (phosphatidylinositol 4-kinase beta), where it controls PI4KB recruitment to the TGN and subsequent phosphatidylinositol-4-phosphate (PtdIns4P) production. As PtdIns4P is essential for AP-4 recruitment, SCAMP5 depletion disrupts AP-4-mediated ATG9A trafficking to presynaptic sites, thereby compromising presynaptic autophagy and subsequent protein turnover. Our findings establish that SCAMP5 coordinates ATG9A-dependent presynaptic autophagy through PI4KB recruitment and PtdIns4P production at the TGN, revealing a novel pathway critical for maintaining presynaptic protein homeostasis.

Na+(K+)/H+ exchanger 6 (NHE6) on synaptic vesicle (SV) is critical for the presynaptic regulation of quantal size at the glutamatergic synapses by converting the chemical gradient (ΔpH) into membrane potential (Δψ) across the SV membrane. We recently found that NHE6 directly interacts with secretory carrier membrane protein 5 (SCAMP5), and SCAMP5-dependent recruitment of NHE6 to SVs controls the strength of synaptic transmission by modulation of quantal size of glutamate release at rest. It is, however, unknown whether NHE6 recruitment by SCAMP5 plays a role during synaptic plasticity. Here, we found that the number of NHE6-positive presynaptic boutons was significantly increased by the chemical long-term potentiation (cLTP). Since cLTP involves new synapse formation, our results indicated that NHE6 was recruited not only to the existing presynaptic boutons but also to the newly formed presynaptic boutons. Knock down of SCAMP5 completely abrogated the enhancement of NHE6 recruitment by cLTP. Interestingly, despite an increase in the number of NHE6-positive boutons by cLTP, the quantal size of glutamate release at the presynaptic terminals remained unaltered. Together with our recent results, our findings indicate that SCAMP5-dependent recruitment of NHE6 plays a critical role in manifesting presynaptic efficacy not only at rest but also during synaptic plasticity. Since both are autism candidate genes, reduced presynaptic efficacy by interfering with their interaction may underlie the molecular mechanism of synaptic dysfunction observed in autism.
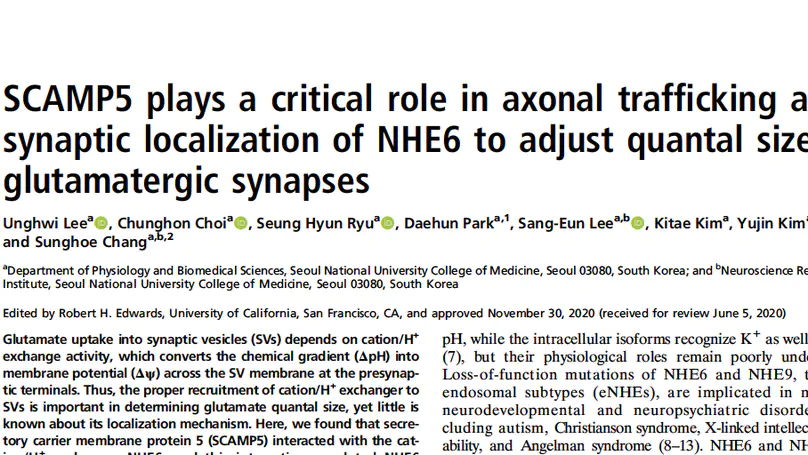
As a vesicular Na+(K+)/H+ exchanger, NHE6 promotes glutamate filling into synaptic vesicles (SVs). Thus, proper localization of NHE6 is critical for determining synaptic strength at gluta- matergic synapses, but the underlying mechanism remains un- known. We found that an SV-enriched protein, SCAMP5, is a key determinant for axonal trafficking and synaptic localization of NHE6. By perturbing their interaction, NHE6 fails to be localized at synaptic sites, resulting in hyperacidification of the SV lumen and a significant reduction in the quantal size of glutamate re- leased. Since both are autism candidate genes, our results sug- gest that impaired interaction between two proteins could relate to the synaptic dysfunction observed in autism.
Recent Publications
News
News & Columns
Contact
- rsh5410@snu.ac.kr
- +82-10-5471-0650
- 103 Daehak-ro Jongno-gu, Seoul, Republic of Korea 03080
- Enter Biomedical Science Building and take the stairs to Room 314 on Floor 3
-
Weekday 09:00 to 18:00
Wednesday 09:00 to 15:00 - Book an appointment
- Zoom Me
- DM Me
![SCAMP5 단백질의 ATG9A 수송 조절을 통한 시냅스 자가포식 조절 기전 [Autophagy]](/talk/scamp5-%EB%8B%A8%EB%B0%B1%EC%A7%88%EC%9D%98-atg9a-%EC%88%98%EC%86%A1-%EC%A1%B0%EC%A0%88%EC%9D%84-%ED%86%B5%ED%95%9C-%EC%8B%9C%EB%83%85%EC%8A%A4-%EC%9E%90%EA%B0%80%ED%8F%AC%EC%8B%9D-%EC%A1%B0%EC%A0%88-%EA%B8%B0%EC%A0%84-autophagy/featured_hu579d4b97ff8eedaca135b7f9d274c528_417081_150x0_resize_q75_h2_lanczos_3.webp)



